Regulatory compliance is no longer a cost—it’s a competitive advantage.
Effective March 16, 2025, EU Regulation 2025/351 overhauled standards for food-contact materials (FCMs) and plastics, introducing stringent rules for reusable personal protective equipment (PPE) labeling and non-intentionally added substances (NIAS). This guide unpacks critical compliance steps and actionable solutions.
1. Reusable PPE Labeling: Balancing Compliance & User Experience
New Article 14a mandates detailed labeling for reusable PPE (e.g., protective suits, masks):
Key Requirements
-
Aging Management Instructions
- Guidelines to slow degradation (e.g., approved cleansers, storage conditions).
- Visual indicators of material failure (e.g., discoloration, cracks).
- Warnings on misuse-induced migration risks.
-
Usage Limitations
- Temperature thresholds and operational limits.
- Maximum reuse cycles (validated by testing).
- Prohibited chemicals (e.g., chlorine-based disinfectants).
-
Innovative Label Formats
- Dynamic QR codes linking to updatable e-manuals.
- ISO pictograms minimizing language barriers.
- Layered design: Core safety data + scannable details.
Actionable Tip: Adapt the EN 14126:2003 protective clothing label framework to integrate digital layers, reducing redesign costs for multi-market compliance.
2. NIAS Limits: Solving the 0.00015 mg/kg Challenge
The regulation defines “high purity” and imposes unprecedented NIAS migration ceilings:
| NIAS Category | Limit | Compliance Strategy |
|---|---|---|
| Positive-list NIAS | List-specific | EFSA-certified supplier documentation |
| Risk-assessed substances | ≤0.05 mg/kg | Substance traceability databases |
| Non-genotoxic unassessed substances | ≤0.05 mg/kg | QSAR screening + zebrafish embryo tests |
| Potentially hazardous unassessed substances | ≤0.00015 mg/kg | Process innovation + molecular capture |
4 Proven Solutions
- Reverse Engineering GC-Orbitrap mass spectrometry to identify contamination sources.
- Process Barriers Molecular sieve layers (e.g., zeolite-modified PP) trapping low-MW by-products.
- Blockchain Verification End-to-end digital traceability from raw materials to recycling.
- AI Aging Models Machine learning predicting NIAS release after 200 reuse cycles.
3. Recycled Plastics Framework: Circular Economy Rules
Recycled materials are permitted under strict conditions:
- Certification: EN 15343 recycling certification + batch contaminant reports.
- Closed-Loop Systems: Mandatory for medical PPE (full traceability required).
- Performance Validation: Post 5 disinfections, meet EN 14126:2003 standards (tear resistance ≥25N, puncture resistance ≥20N).
Case Study: A German manufacturer added 0.8% nano-TiO₂ masterbatch, boosting recycled PP’s aging resistance by 300% for face shields (ECHA-approved).
4. 3-Phase Compliance Roadmap
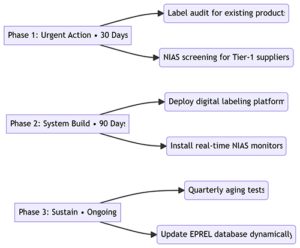
- Dec 16, 2025: Submit compliance declarations for transitional products.
- Sept 16, 2026: Exhaust pre-regulation stock.
- 2026 onward: Annual third-party NIAS audits.
5. Future Regulatory Trends
Upcoming EU developments demand proactive preparation:
- 2026: Digital Product Passports (DPP) mandated for reusable PPE.
- 2027: EN 17353 standard for PPE aging assessment (draft in progress).
- Enforcement: Portable DESI spectrometers for on-site NIAS checks.
Conclusion
EU 2025/351 transforms compliance into market advantage. Early adopters of smart labeling and NIAS control will secure brand premium (up to 15% by industry estimates) and consumer trust.
Act now: The September 16, 2026, stock depletion deadline leaves no room for delay. Turn your labels into stories of safety—your passport to the European market.
Regulations are the language of business; compliance is the wisdom engraved by champions.
Information current as of June 30, 2025. Based on Regulation (EU) 2025/351, Food Packaging Forum, and European Commission guidelines. Technical solutions require product-specific validation.








